![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
Exponential Decay - Example
Problem: A radioactive isotope of iodine decays exponentially. There is 50 mg of the element initially, 35 mg after 4 days.
(a) Find a formula of the form
![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
(b) Find the half-life,
![]() , of the isotope.
, of the isotope.
 |
 |
 |
 |
As we found out, the formula decsribing the decay is
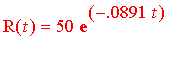 .
.
Half-life is the time,
![]() , after which half of the initial amount of 50 mg decomposes, and 25 mg is left. Hence, to find
, after which half of the initial amount of 50 mg decomposes, and 25 mg is left. Hence, to find
![]() we have to solve for
we have to solve for
![]() the equation
the equation
![25 = 50*exp(-.891e-1*t[h])](images/decsf7.gif) .
.
We divide both sides by 50, and take the natural logarithm of both sides. We obtain
![ln(25/50) = ln(exp(-.891e-1*t[h]))](images/decsf8.gif) .
.
Since
![ln(exp(-.891e-1*t[h])) = -.891e-1*t[h]](images/decsf9.gif) (from properties of logs), we get
(from properties of logs), we get
![-.891e-1*t[h] = ln(1/2)](images/decsf10.gif) .
.
Hence, the half-life is
![t[h] = ln(1/2)/(-.891e-1)](images/decsf11.gif) .
.
The latter gives the half-life
![]() days, approximately.
As we know, that means that half of the amount will be gone after 7.78 days; after the next 7.78 days half of what's left will be gone again, and so on.
days, approximately.
As we know, that means that half of the amount will be gone after 7.78 days; after the next 7.78 days half of what's left will be gone again, and so on.