![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
Exponential Decay - Example
Problem: A radioactive isotope of iodine decays exponentially. There is 50 mg of the element initially, 35 mg after 4 days.
(a) Find a formula of the form
![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
(b) Find the half-life,
![]() , of the isotope.
, of the isotope.
 |
 |
 |
 |
We know already that our formula is
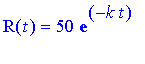 .
.
To find k , we use the fact that after 4 days, that is, at t=4 , there are 35 mg of iodine left. That gives us the following equation
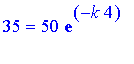 .
.
We divide both sides by 50, and take the natural logarithm of both sides. We obtain
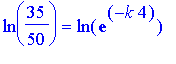 .
.
Since
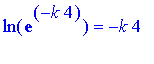 (from properties of logs), we get
(from properties of logs), we get
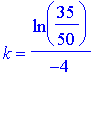 = 0.0891
.
= 0.0891
.
Hence, the formula describing the decay of iodine is
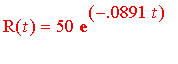 .
.