![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
Exponential Decay - Example
Problem: A radioactive isotope of iodine decays exponentially. There is 50 mg of the element initially, 35 mg after 4 days.
(a) Find a formula of the form
![R(t) = R[0]*exp(-k*t)](images/decay1.gif)
(b) Find the half-life,
![]() , of the isotope.
, of the isotope.
 |
 |
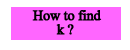 |
 |
As in every applied problem, first you have to define your variables and their units. Let
![]() denote the amount of iodine left, in mg, after time
t
. Time
t
is measured in days after the initial moment when we began observing the isotope.
To find the formula
denote the amount of iodine left, in mg, after time
t
. Time
t
is measured in days after the initial moment when we began observing the isotope.
To find the formula
![R(t) = R[0]*exp(-k*t)](images/decsa4.gif)
describing the amount left as time changes, we need to find the constants
![]() and
k
.
and
k
.